CLIP‐170 spatially modulates receptor tyrosine kinase recycling to coordinate cell migration - Zaoui - 2019 - Traffic - Wiley Online Library
Overexpression of the microtubule-binding protein CLIP-170 induces a +TIP network superstructure consistent with a biomolecular condensate | PLOS ONE

Structural basis for tubulin recognition by cytoplasmic linker protein 170 and its autoinhibition | PNAS

Ninein is essential for apico-basal microtubule formation and CLIP-170 facilitates its redeployment to non-centrosomal microtubule organizing centres | Open Biology

The microtubule plus-end-tracking protein CLIP-170 associates with the spermatid manchette and is essential for spermatogenesis

The microtubule plus-end-tracking protein CLIP-170 associates with the spermatid manchette and is essential for spermatogenesis

Arsenic trioxide disturbs the LIS1/NDEL1/dynein microtubule dynamic complex by disrupting the CLIP170 zinc finger in head and neck cancer - ScienceDirect
Model for plus-end tracking activity of mammalian EB1 and CLIP-170. (A)... | Download Scientific Diagram
CLIP-170S is a microtubule +TIP variant that confers resistance to taxanes by impairing drug-target engageme
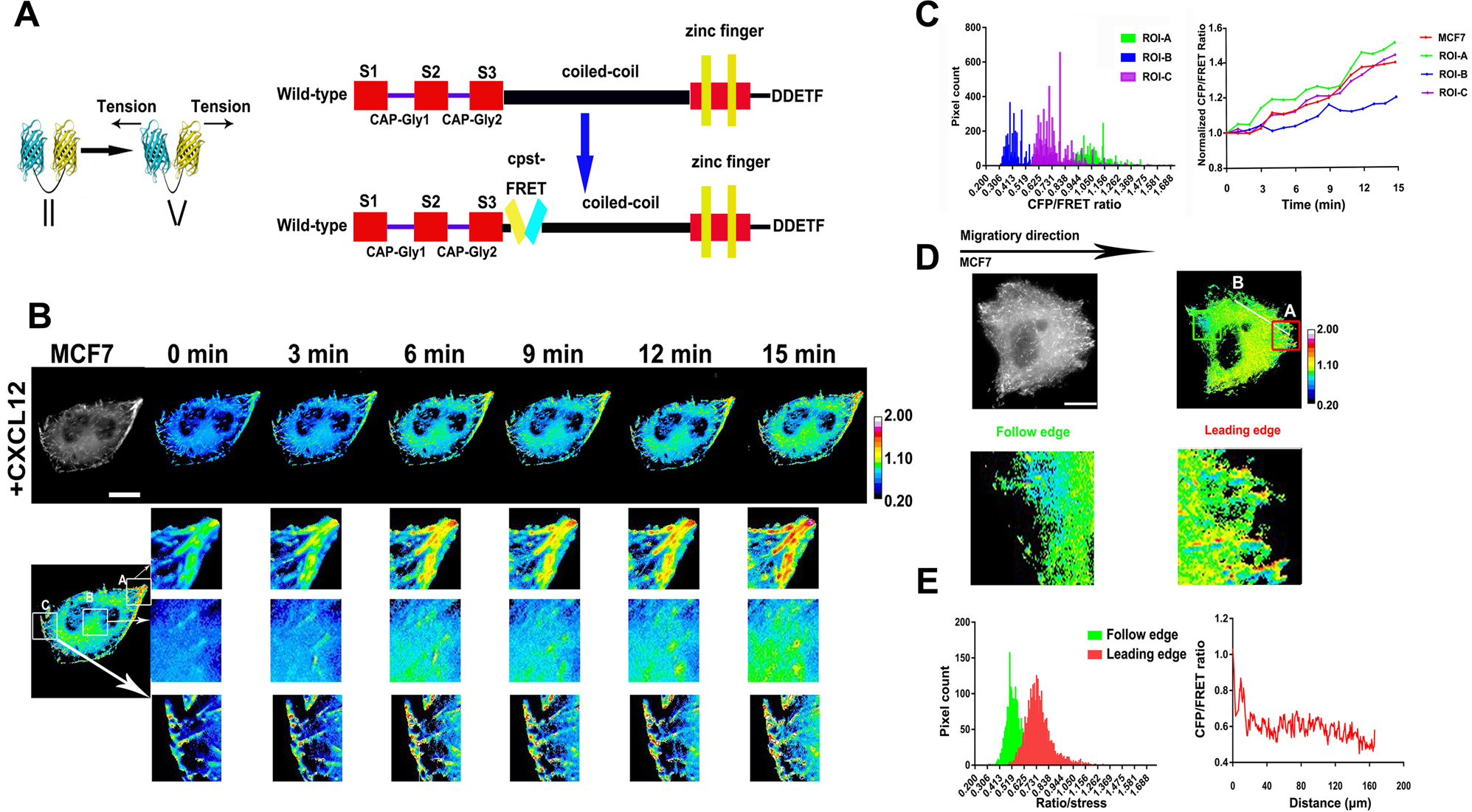
Tension of plus-end tracking protein Clip170 confers directionality and aggressiveness during breast cancer migration | Cell Death & Disease

The microtubule plus-end-tracking protein CLIP-170 associates with the spermatid manchette and is essential for spermatogenesis
Overexpression of the microtubule-binding protein CLIP-170 induces a +TIP network superstructure consistent with a biomolecular condensate | PLOS ONE

Microtubule binding proteins CLIP-170, EB1, and p150Glued form distinct plus-end complexes - ScienceDirect

HIV‐1 capsids mimic a microtubule regulator to coordinate early stages of infection | The EMBO Journal
Potential mechanisms of microtubule plus-end tracking. (A) Motor-driven... | Download Scientific Diagram

CLIP-170S is a microtubule +TIP variant that confers resistance to taxanes by impairing drug-target engagement - ScienceDirect

Overexpression of the microtubule-binding protein CLIP-170 induces a +TIP network superstructure consistent with a biomolecular condensate | bioRxiv