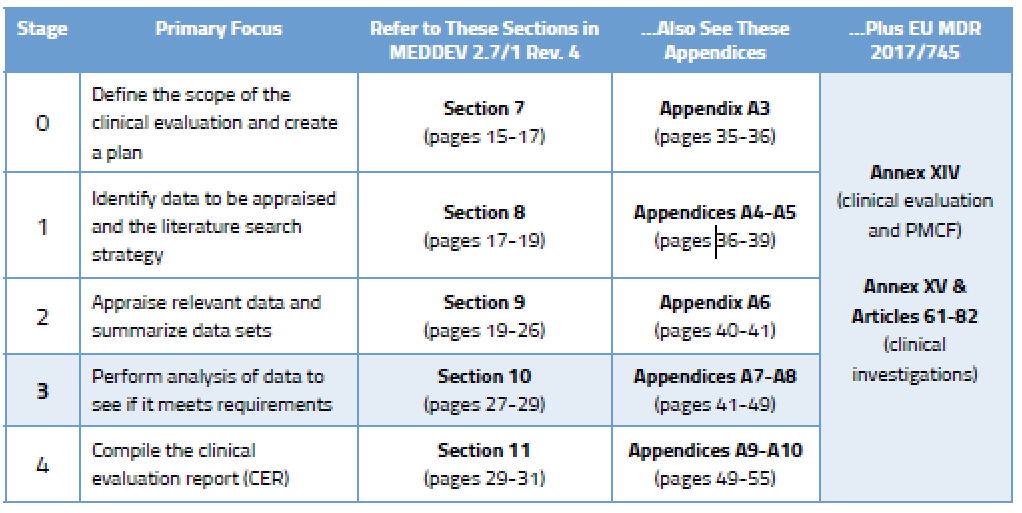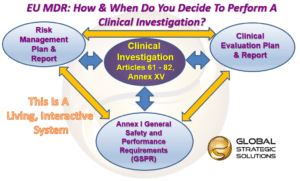
What Is The Difference Between Clinical Evaluation and Clinical Investigation? | Global Strategic Solutions

Issue 3: Resource Considerations and Necessary Organization and Structure of Clinical Investigation | Resources for Clinical Investigation: Report of a Study | The National Academies Press

Issue 3: Resource Considerations and Necessary Organization and Structure of Clinical Investigation | Resources for Clinical Investigation: Report of a Study | The National Academies Press